About princess®
Threads
(CE 2292)
- Absorbable PDOs for effective skin rejuvenation, reinforcement and lifting
- Safe & easy alternative for light surgical lifting
- Immediate and long lasting effect
- Minimal downtime

About princess®
Threads
(CE 2292)
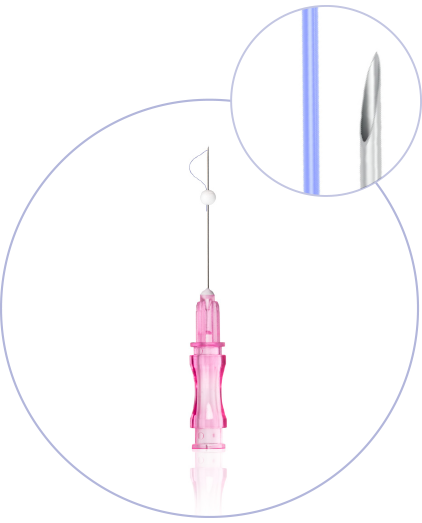
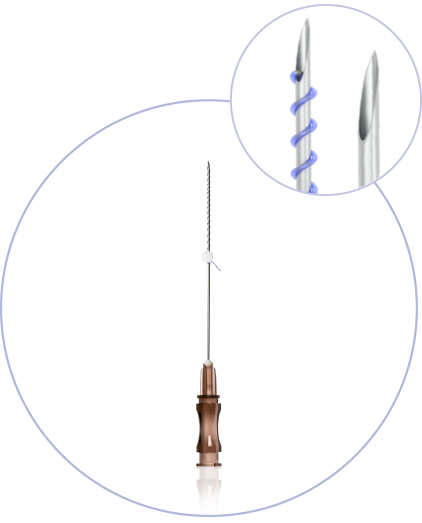
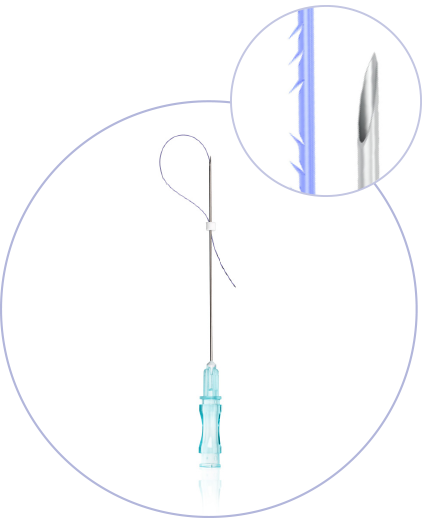
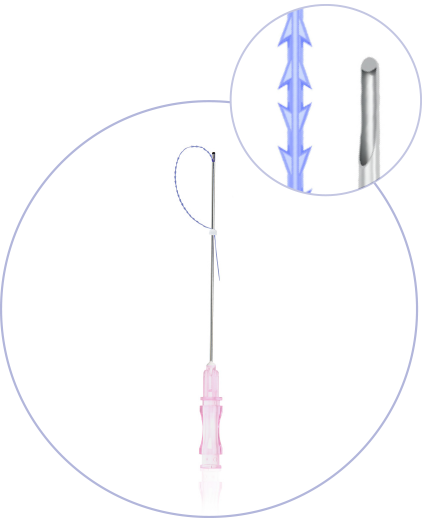
What makes princess®
Threads special?
(CE 2292)
Clinically proven absorbable polydioxanone (PDO) 1-3
Long lasting result (up to 1 year) 4
Simple choice of the appropriate suture for different anatomical zones
Ergonomic hub for easy and fast application
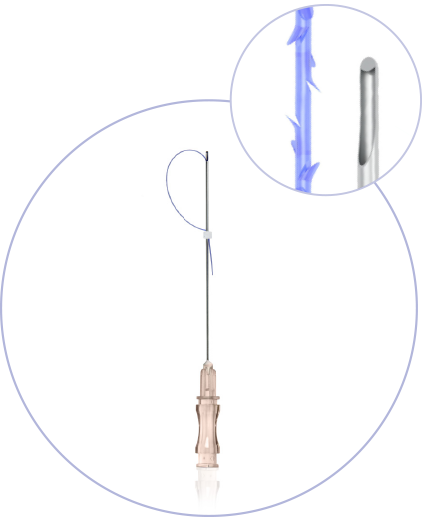
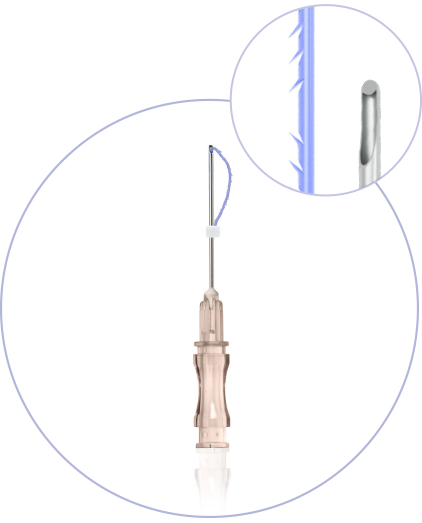
Ultra-Thin Wall needle/cannula enables using thicker and stronger suture
Needles with sharp cutting angle to minimize the pain, traumatisation, and downtime
Special coating of the needle / cannula enables the smooths application
Automated, cutting edge and clean production process
CE Mark certified
Effects
Mechanism of action
The maintenance of the aesthetic outcome over months after the reabsorption of the PDO sutures is due to the foreign body reaction (induced by the PDO suture), the enhanced collagen proliferation and the stimulation of fibroblasts at the place of implantation.
When inserted into the skin, the threads act as a ‘scaffold’ that helps to ‘hold’ the skin against the effects of gravity.
Tissue elevation is the immediate effect, achieved thorough mechanical action by the suture.
References
The medical practitioner confirms having informed the patient of a likely risk associated with the use of the medical device in line with its intended use. For risks and adverse events associated with the use of the product consult the instructions of use.
Distributed by
Croma-Pharma GmbH
Industriezeile 6
2100 Leobendorf
Tel.: +43/ 2262/ 68468 - 0
Fax: +43/ 2262/ 68468 - 165
E-Mail: office@croma.at
Patented and certified by GRAND AESPIO INC.
2F, 41 Hakdong-ro 3-gil, Gangnam-gu, Seoul 06043 Korea
Produced by MEDIFIRST Co.,Ltd.
#1049-16, Charyeonggogae-ro,
Gwandeok-myeon, Dongnam-gu,
Cheonan-si, Chungcheongnam-do,
31223, Republic of Korea
European Representative:
Intermundien-lemon Europe GmbH
Brueckster, 47
D-44787 Bochum, Germany
PTHHP0323